Physicians
The Vent-Os System achieved post-procedural patency in 95 percent of the sinus ostia treated in a multi-center study and submitted as part of the Company’s FDA application; five percent of the treated ostia could not be visualized. In the study, the Vent-Os device was inserted into the maxillary sinus opening at the beginning of the procedure and removed after 60 minutes. No adverse events occurred during insertion or removal of the device. Three-month follow-up was completed in 33 patients (55 ostia), with 93 percent of the treated ostia remaining patent and seven percent that could not be visualized. No ostia were reported to be occluded. At 6 months, 30 patients were available for follow-up and 45/49 (92%) of treated ostia were visibly patent with 4/49 (8%) “indeterminate”; no maxillary ostia were clinically non-functional. At 12 months, 27 patients with 45 treated ostia were available for evaluation, with 93 percent of ostia (42) visibly patent and seven percent (3) unable to be visualized. All ostia were clinically functional1
Fifteen percent of patients in the study were treated in an office setting after pre-procedural injection of anesthesia; no additional anesthesia or medication was required for these patients for the duration of the procedure. This is in contrast to balloon dilation devices, which often require administration of anxiolytics, analgesics and/or additional local injections of anesthetics during the procedure to increase patient tolerability. The remaining patients were treated in the operating room adjunctive to functional endoscopic sinus surgery (FESS).
The Vent-Os® Sinus Dilation System is an instrument intended to provide a means to access the sinus space and to dilate the maxillary sinus ostia and associated spaces in adults for diagnostic and therapeutic procedures.
(1) Amin R Javer, Peter Catalano, James Gordon and Hesham Saleh. Long term outcome analysis of low pressure osmotic dilation of the maxillary ostia: 12 months follow up. 2014 Annual Meeting of the American Rhinologic Society (abstract #781).

Getting Started is Simple!
Our VOICE Program makes it easy to bring sinus dilation into your practice!
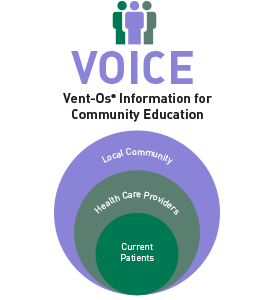
The VOICE Program from SinuSys Corporation is an effective and efficient way to educate your patients, colleagues, and local community about your offering of the Vent-Os procedure for sinusitis. Specifically designed for ENT practices, the VOICE Program consists of customizable programs for use in your office and online.
As a VOICE (Vent-Os® Information for Community Education) program participant, you will have access to customizable, professionally produced educational tools, designed for immediate use over a range of communication channels.
Reach out to your patient community
- At the point of care with in-office patient education materials
- With direct-to-patient mailings and newsletters, using email and traditional mail
- Over the Internet with search-optimized website content
- Through social media with focused educational postings
- Through the local media with print awareness campaigns and/or press releases
Make it easy for patients to find you
All VOICE participants are automatically listed in the Find-A-Physician directory on the SinuSys patient information web site.
We’ll help you get started
To ensure your success with VOICE, a SinuSys team member can train you or a designated member of your practice on the customization and implementation of materials and programs best suited to your professional goals and practice.

Physicians
More Clinical Information
Contact Us
- 2468 Embarcadero Way
Palo Alto, CA 94303 - Toll Free: 1-855-4-SinuSys (474-6879)
- Office: 650-213-9988
- E-Fax: 650-213-9688
- Customer Service
- Contact Us Now